
Determining Oxidation Numbers Worksheet
2. The oxidation number of a monatomic ion equals the charge on the ion. 3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in a compound is always -1. 5. Oxygen has an oxidation number of -2 unless it is combined with F (when.
Oxidation Number Worksheet Answer Key
Give the oxidation number of each kind of atom or ion. sulfate b. Sn c. S2- d. Fe3+ e. Sn4+ f. nitrate g. ammonium Calculate the oxidation number of chromium in each of the following. Cr2O3 b. Na2Cr2O7 c. CrSO4 d. chromate e. dichromate
16 Best Images of Organic Oxidation Reactions Worksheet Balancing
Liveworksheets transforms your traditional printable worksheets into self-correcting interactive exercises that the students can do online and send to the teacher.. Chemistry (1061818) Main content: Oxidation number (1946116) finding the oxidation number. Loading ad. Share / Print Worksheet. Google Classroom Microsoft Teams Facebook.

Aluminum Aluminum Oxidation Number
A monoatomic ion has an oxidation number equal to its charge. For example, the oxidation number of the oxygen in the oxide ion, O 2-, is -2. The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion. Let's examine the oxidation numbers of some common elements. Notice the periodic trend among the main-group.
Charting Oxidation Number Worksheet Answer Key Worksheets Joy
The number of valence electrons on an atom is equal to its group number. In a cation, the oxidation number is equal to the number of these electrons which have been removed. Transition metal cations have a configuration dz where Z is the number of valence electrons left over after ionization: Z = number of valence electrons on atom- charge of.

Oxidation Numbers Worksheet
Oxidation numbers Student worksheet: CDROM index 30SW Discussion of answers: CDROM index 30DA Topics Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of assigning oxidation numbers - electronegativity values and oxidation number rules. Level Very able post-16 students.
Worksheet 2. Oxidation Numbers Worksheet 2
One way of reflecting this is through changes in assigned oxidation numbers. Oxidation numbers are real or hypothetical charges on atoms, assigned by the following rules: Atoms in elements are assigned 0. All simple monatomic ions have oxidation numbers equal to their charges. (e.g., all Group 1 ions are +1; all group 2 ions are +2; all the.

Worksheet Oxidation Numbers Answer Key
a. The charge on all free elements is zero. b. The charge on all metals of group 1 of the periodic table is +1 c. The charge on all metals of group 2 of the periodic table is +2 d. The charge on aluminum is +3 e. The charge on hydrogen is +1, except in hydrides where it is -1 f.

Worksheet Assigning Oxidation Numbers Key.doc
The Assigning Oxidation Numbers to Elements Worksheet consists of two pages:Page 1: - Students will write the SIX (6) Rules for determining the Oxidation Number to Monotamic (Unreacted) Elements, Elements in a Compound, and Elements in a Polyatomic Ion.- THREE (3) Practice Problems in which students must determine the Oxidation State of.

Oxidation Number Worksheet 1Write the rule next to your answer
Purpose: This exercise is designed to teach the student how to assign oxidation numbers. Oxidation numbers are very important and are used for 1) naming compounds, 2) balancing oxidation-reduction reactions, 3) calculations in electrochemistry and other areas of chemistry. Exercises - Give the oxidation number for the following atoms: 2 O O =

️Oxidation Numbers Worksheet And Answers Free Download Goodimg.co
The oxidation number of any uncombined element is 0. The oxidation number of a monatomic ion equals the charge on the ion. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. The oxidation number of fluorine in a compound is always -1
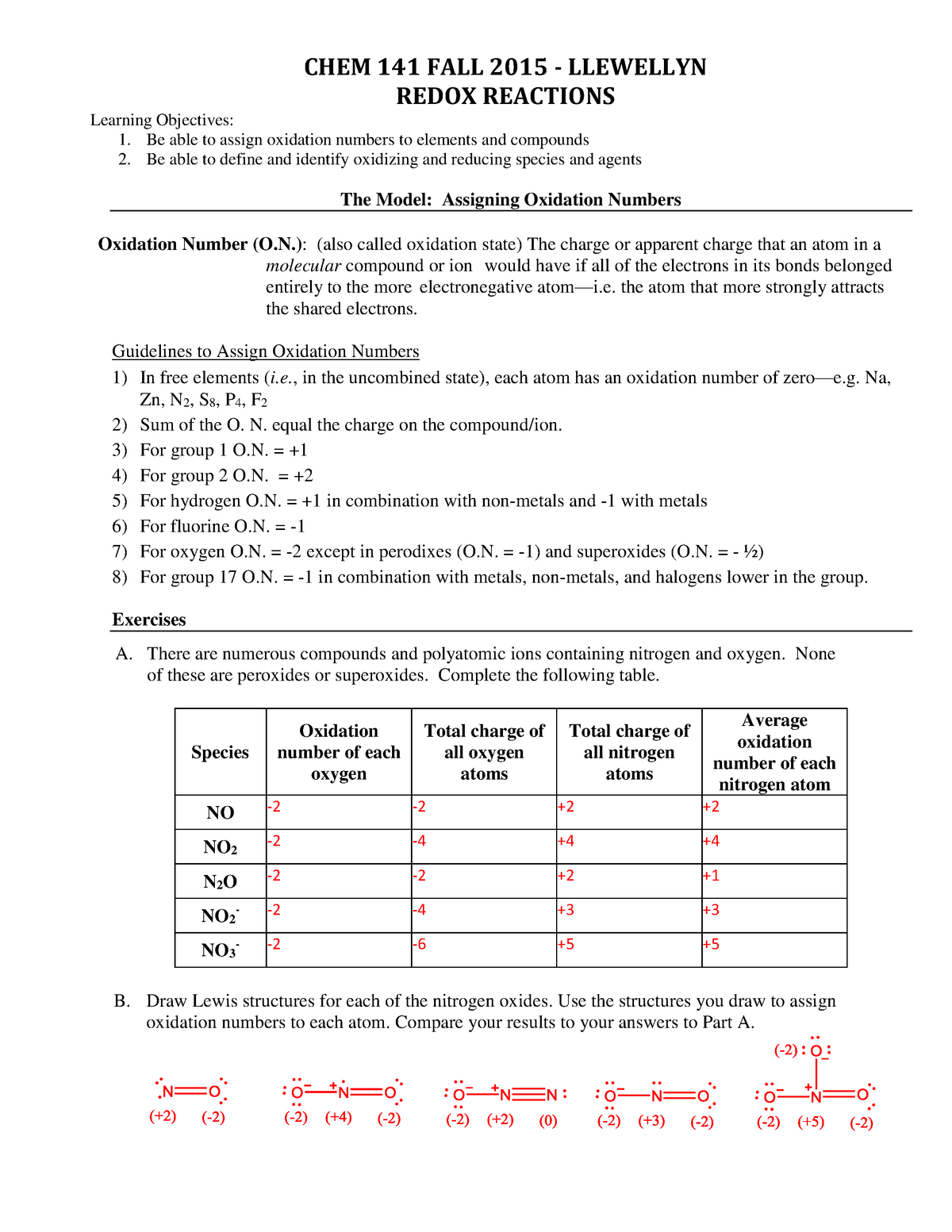
Assigning Oxidation Numbers Worksheet Answer Key Escolagersonalvesgui
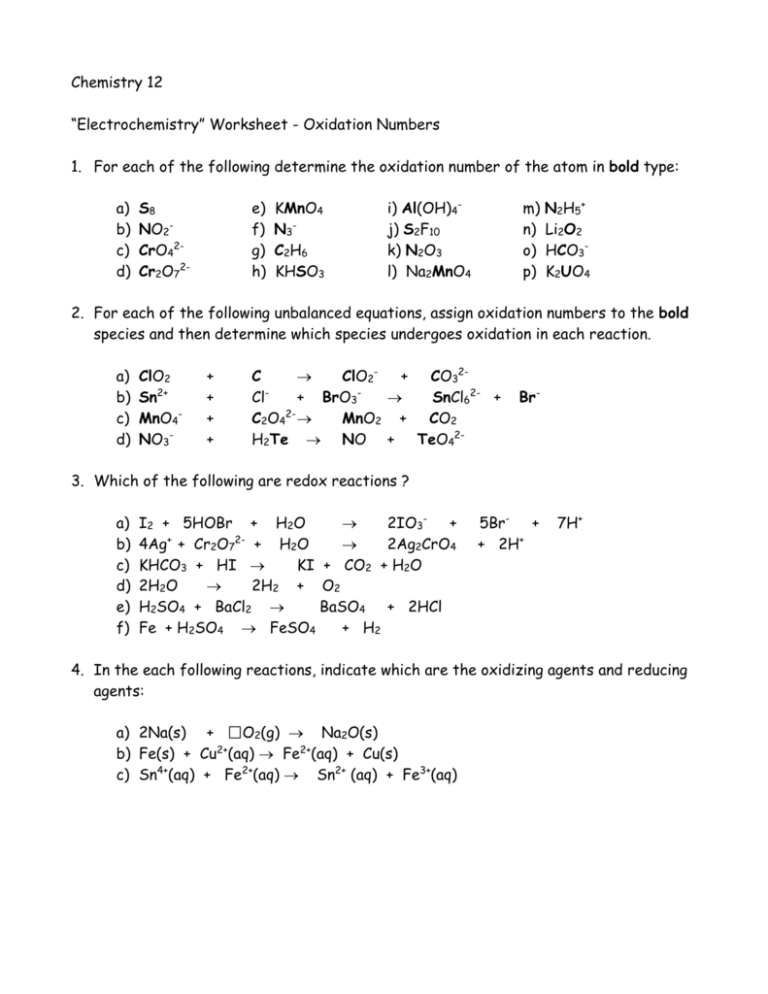
ASSIGNING OXIDATION NUMBERS WORKSHEET Part A: In the following questions, give the oxidation number of the indicated atoms/ion. N in N2O3 __________ S in H2SO4 __________ C __________ C in CO __________ Na in NaCl __________ H in H2O __________ Ba in BaCl2 __________ N in NO2 - __________ S in Al2S3 __________ S in HSO4 - __________
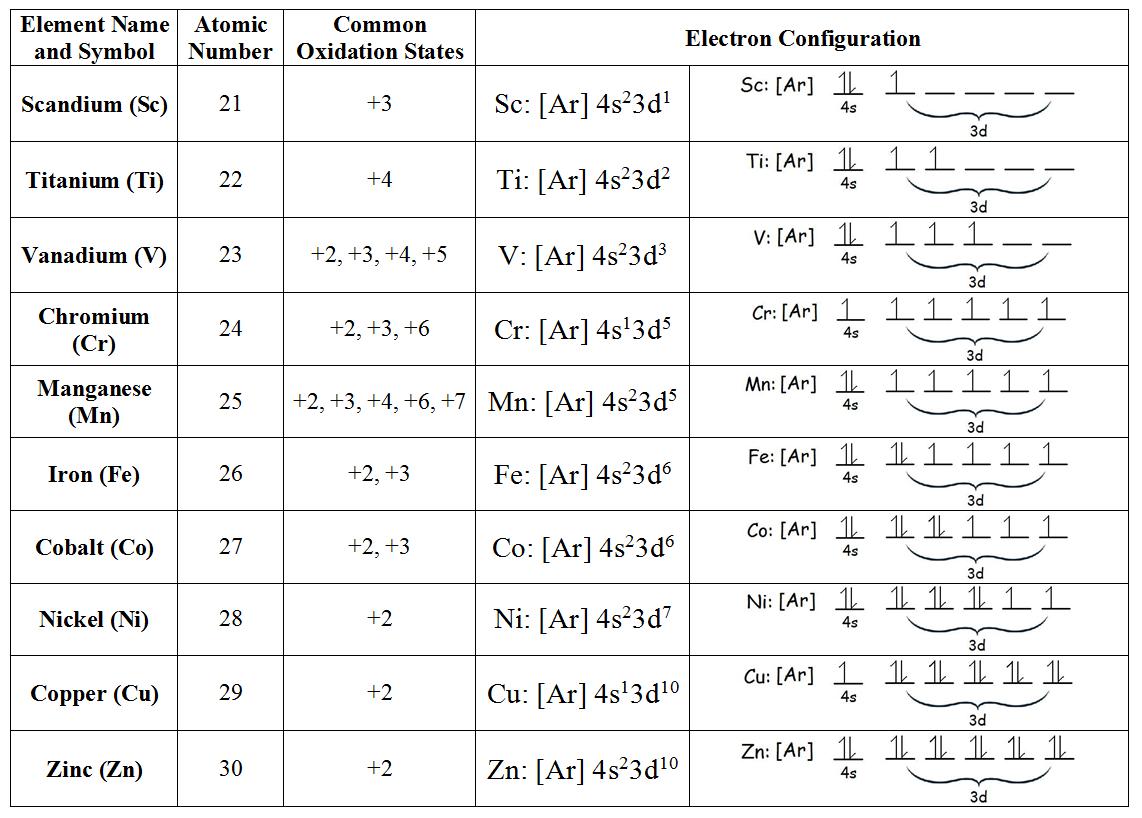
why do transition metals have multiple oxidation states
The oxidation number of oxygen in most compounds is \(-2\). The oxidation number of hydrogen in most compounds is \(+1\). The oxidation number of fluorine in all compounds is \(-1\). Other halogens usually have an oxidation number of \(-1\) in binary compounds, but can have variable oxidation numbers depending on the bonding environment.

Using Oxidation numbers to find formulas (polyatomic ions) worksheet
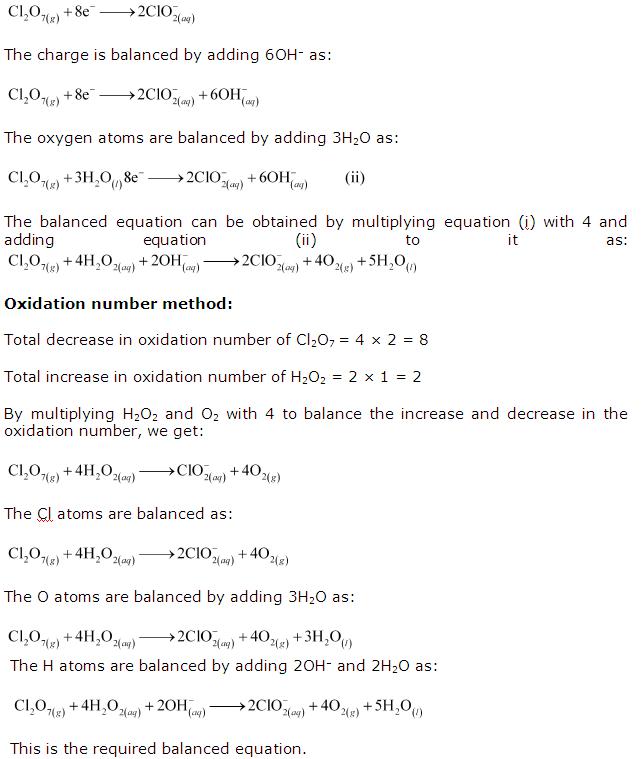
Reduction 1⁄2 Reaction: 0 Br2 + 2e ̄ à 2Br ̄. Now, combine the new half-reactions into a final equation. Note that all of the electrons have cancelled out: New Oxidation 1⁄2 Reaction: 2K0 à 2K+ + 2e ̄. Reduction 1⁄2 Reaction: + Br2 0 + 2e ̄ à 2Br ̄. Balanced Ionic Equation: 2K0 + Br2.

Oxidation Numbers Worksheet With Answers
Worksheets: General Chemistry (Traditional)

ASSIGNING OXIDATION NUMBERS WORKSHEET
Mind Matters Pedagogy-Science Resources. With this Colour-by-Number worksheet, chemistry or science students can practice calculating oxidation numbers / oxidation states as part of the Oxidation -Reduction (RedOx Unit) in a fun and relaxing way which also improves memory. Students will first calculate the oxidation numbers of the underlined.